Meet New MoCRA Adverse Events Requirements
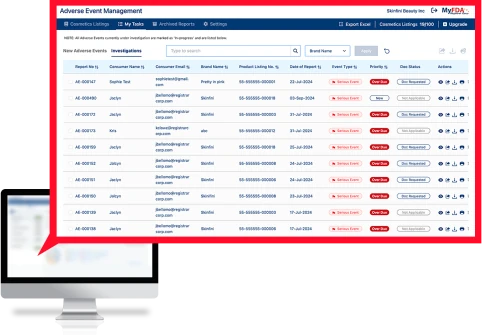
Registrar’s Adverse Events Management (AEM) software is a turnkey solution to manage the entire Adverse Event process from consumer intake to investigation to FDA reporting and records storage.
Adverse Events 101 in just 2 minutes.
Registrar’s Adverse Events Management (AEM) software is a turnkey solution to manage the entire Adverse Event process from consumer intake to investigation to FDA reporting and records storage.

MoCRA has New, Stringent Requirements
for Adverse Events:
MoCRA has New, Stringent Requirements for Adverse Events:

- Display contact information on both the primary and secondary packaging
- Collect detailed personal and medical information from the consumer
- Investigate to determine if the adverse event is a “serious” event
- Report “serious” adverse event to the FDA within 15 business days
- Record all health-related adverse events and maintain records for up to 6 years
Purpose-built for cosmetic Adverse Events
Purpose-built for
cosmetic Adverse Events
The Adverse Events Management (AEM) software securely intakes sensitive consumer medical data, tracks all adverse events for all products globally, transmits information to internal stakeholders, and formats serious adverse events to the FDA MedWatch format for submission to the FDA.




Consumer-friendly Intake
Consumer fills digital form to confidentially submit up to 43 fields of data through web link or QR code on product label.

Instant Alert & Fast Response
Monitor adverse events globally for every SKU in real-time and get the earliest indicator of potential safety or quality issues.
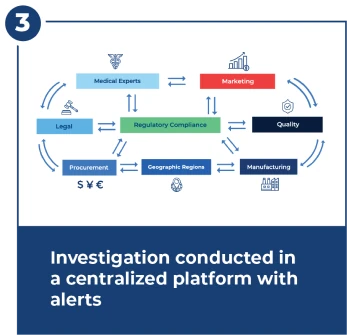
Investigate Within 15 Day FDA Deadline
Investigations are complex and require close and fast coordination across many teams. The investigation center provides workflows, alerts, dashboards, and centralized document management.

Partnering with Denver Health to streamline adverse event reporting and regulatory compliance.

Real-time Alerts

Workflows

Dashboards

Document Management
FDA MedWatch Ready
Serious adverse event reports pre-formatted to the MedWatch 3500A for quick submission.

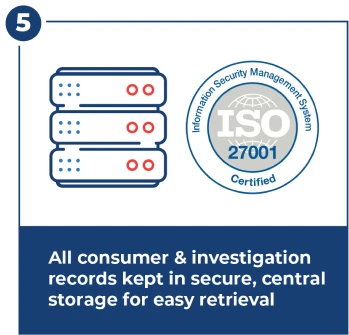
World-Class Data Security
ISO 27001-certified security for storage and transmission of medical and personal identifiable information (PII).

Your Guide to MoCRA Adverse Events
Executive Brief Covers:
- 5 key requirements for Adverse Events
- Business and legal risks of non-compliance
- Gaps in existing processes
- Recommendations for meeting requirements
Are you Sure You’re MoCRA Compliant?
Schedule a short call with our Adverse Events experts.
Are you Sure You’re Compliant?
Schedule a short call with our
Adverse Events experts.
All You Need to Know About Adverse Events
All You Need to Know
About Adverse Events
Learn from our cosmetic experts on how to navigate MoCRA adverse events reporting compliance.

Complete Adverse
Events Guide


