Do I need FDA Approval? Achieve FDA Compliance with Registrar Corp
Learning how to get FDA approval—or determining if you even need it—depends entirely on the type of product you plan to market in the U.S. Many assume all products must be reviewed and approved before entering the market, but the reality is far more nuanced. FDA does not require all products to be approved before entering the market, and in some cases, only certain components, such as specific ingredients, must undergo the process. Misunderstanding these distinctions can lead to costly delays, labeling violations, and even enforcement actions.
To make informed decisions, companies need more than definitions—they need to understand the regulatory pathways, the stakes of noncompliance, and the consequences of acting on myths. This guide walks through each major product category FDA regulates, explaining when approval is required, when it isn’t, and what compliance steps still apply. It also addresses common misconceptions, provides a quick-reference chart, and links to authoritative FDA resources. Throughout, one core truth holds: FDA registration is not the same as FDA approval, and assuming otherwise can be a costly mistake.
Quick-Reference: FDA Approval & Registration Requirements
| Category | FDA Approval Required? | Registration Required? | Key Exceptions |
| Food & Beverages | No | Yes (most facilities) | New food additives |
| Dietary Supplements | No | Yes (most facilities) | Disease claims trigger drug regulations |
| Drugs | Sometimes | Yes | Conforming OTC monographs don’t require approval |
| Medical Devices | Class III only | Yes | Certain Class I & II require 510(k) clearance |
| Cosmetics | No (except color additives) | Yes (under MoCRA) | Claims to affect structure/function can trigger drug regulations |
| Color Additives | Yes | N/A | Some require batch certification |
FDA Approval of Food, Beverages, and Dietary Supplements
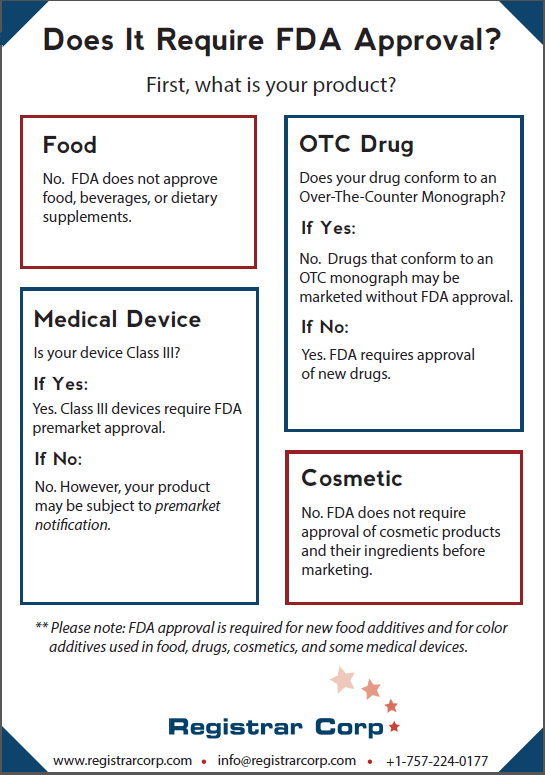
Contrary to popular belief, FDA does not approve food, beverages, or dietary supplements before they are sold in the United States. Food facilities do not need certification or premarket approval, but most must register with FDA and comply with the Food Safety Modernization Act (FSMA).
Registration serves two primary purposes: it gives FDA the necessary contact information for both routine and emergency communications, and it informs the agency about the products and activities at the facility. This information allows FDA to set inspection priorities based on potential food safety risks, focusing attention on higher-risk operations while still conducting randomized checks. For example, a facility producing ready-to-eat foods may be inspected more frequently than a low-risk commodity processor.
FDA enforces these requirements by inspecting registered facilities, issuing warning letters for violations, and detaining shipments that appear noncompliant. Registration also enables targeted outreach during recalls or public health emergencies. However, while registration is required for most food and supplement facilities, it should not be confused with product approval. This distinction is critical across all FDA-regulated industries.
In addition, FSMA mandates that facilities implement and maintain a food safety plan overseen by a Preventive Controls Qualified Individual (PCQI). Completing a recognized PCQI training program helps ensure the responsible person is equipped to identify hazards, validate controls, and manage compliance. Without a designated PCQI, companies risk enforcement actions and delays in resolving inspection findings.
One of the few exceptions in this category is food additives. Unless it is generally recognized as safe (GRAS), any new additive must be approved through a food additive petition, which requires rigorous testing and safety data under the FD&C Act.
Intended use matters: when a product crosses the line from nutrition to therapeutic claims, it may enter a new regulatory category altogether—one that does require approval. For example, dietary supplements that claim to treat, diagnose, prevent, or cure diseases may be regulated as drugs and would then be subject to the approval process outlined in the next section.
Busting the Myth: “FDA approved” foods do not exist. Even if a facility is registered and fully compliant, neither the facility nor its products have been officially approved unless specific ingredients, such as additives, have gone through the approval process.
FDA Approval of Drug Products: Understanding How to Get FDA Approval
Whether a new drug requires FDA approval depends on whether it conforms to an over-the-counter (OTC) monograph. OTC monographs detail specific conditions under which a drug is deemed safe and effective without premarket approval. Final monographs function as “recipes” for drug categories, specifying ingredients, dosages, labeling, and testing standards. FDA also exercises enforcement discretion for certain drugs under tentative final monographs.
If a drug does not conform to an OTC monograph, it must undergo premarket approval. This process involves laboratory analysis, animal studies, and phased human clinical trials, followed by submission of data to FDA. The agency reviews the data to determine whether the benefits outweigh the risks for the intended use. Marketing a non-conforming drug without approval is prohibited under the Food, Drug, and Cosmetic Act (FD&C Act). FDA does not approve compounded drugs.
Drug establishments must register and list their products with FDA to give the agency oversight for inspection and compliance monitoring. Again, this registration and listing process, while necessary, does not imply that FDA has approved the drug or the establishment.
Much like drugs, medical devices follow a tiered path to market—though the criteria and approval thresholds differ.
Busting the Myth: Many believe that FDA must approve all drugs before they are marketed. In reality, if a drug conforms to an OTC monograph, it can be legally marketed without premarket approval.
FDA Approval of Medical Devices: How to Get FDA Approval for Class III Products
FDA classifies medical devices into three categories based on risk: Class I (low risk), Class II (moderate risk), and Class III (high risk). Class III devices, such as pacemakers or certain implants, require premarket approval, which demands robust evidence of safety and effectiveness through clinical trials and technical documentation.
Class I and II devices, unless exempt, must submit a premarket notification (510(k)) demonstrating substantial equivalence to a legally marketed device. This involves providing detailed product specifications, performance data, and sometimes clinical evidence. If FDA determines equivalence, it “clears” the device for marketing rather than “approving” it. As with other categories, device registration and listing requirements help FDA monitor industry activity, but do not equate to product approval.
Busting the Myth: “FDA cleared” and “FDA approved” are not interchangeable. Of those subject to FDA review, most devices are cleared via the 510(k) process, not approved. Only Class III devices require full premarket approval.
FDA Approval of Cosmetics
FDA does not require cosmetic products or their ingredients to be approved before they go to market, except for color additives. Prior to December 2022, cosmetic companies were not required to register with FDA at all. Under the Modernization of Cosmetic Regulations Act (MoCRA), companies must now register their facilities and list their products.
Before MoCRA, FDA oversight relied heavily on post-market enforcement—intervening when products were found unsafe or misbranded. Now, with mandatory registration, FDA has better visibility, can assess compliance proactively, and schedule inspections based on risk profiles.
It’s important to note that registration is not the same as approval. The misconception persists that FDA registration indicates endorsement of a product, when in fact it simply grants FDA the authority to inspect and monitor compliance. Additionally, cosmetic claims that imply therapeutic benefits—such as curing or preventing disease—can cause FDA to regulate the product as a drug, which may require approval.
Busting the Myth: Cosmetic registration under MoCRA does not mean FDA has approved the product. Approval applies only to specific color additives or if the product is regulated as a drug.
One key exception to this non-approval model is color additives—whether used in cosmetics, food, or drugs—which are regulated under their own approval process.
FDA Approval of Color Additives
FDA must approve color additives used in food, drugs, cosmetics, and certain medical devices. This approval ensures the additive meets safety standards for its intended use. Certain high-risk colors require batch certification for every lot before use.
Color additives may only be used according to their approved specifications and restrictions. Products containing unapproved color additives are considered adulterated under the FD&C Act and may be detained at U.S. ports of entry.
Busting the Myth: Approval of a color additive for one use does not permit it to be used in other product categories. Each approved use has specific limitations and conditions.
Labeling FDA Approved Products
Even for approved products, there’s another area that trips up manufacturers: labeling. Manufacturers of drugs and devices that require approval may include “FDA Approved” on product labeling if they have received written confirmation from the agency. The FDA logo, however, may not be used, as it implies endorsement and can lead to civil or criminal liability. Regardless of approval status, all regulated facilities must comply with Current Good Manufacturing Practices (CGMPs) and labeling requirements.
For products requiring approval or clearance, such as certain drugs and devices, FDA also reviews and approves the product’s labeling as part of the process. However, in most other cases, labels are not pre-approved by FDA.
FDA enforces compliance through facility inspections, product sampling, and shipment checks at the border.
Work Toward FDA Approval With Registrar Corp
Registrar Corp helps food, beverage, drug, medical device, and cosmetic companies navigate U.S. FDA regulations. We assist with facility registration, product listing, label review, and color additive batch certification. Acting before a compliance deadline or enforcement action can prevent costly delays and loss of market access.
For immediate assistance and to ensure you meet all applicable requirements, call +1-757-224-0177 or chat with a Regulatory Advisor 24/7.









