CHALLENGE
Inaccurate monitoring that exposed client to costly penalties and missing suppliers
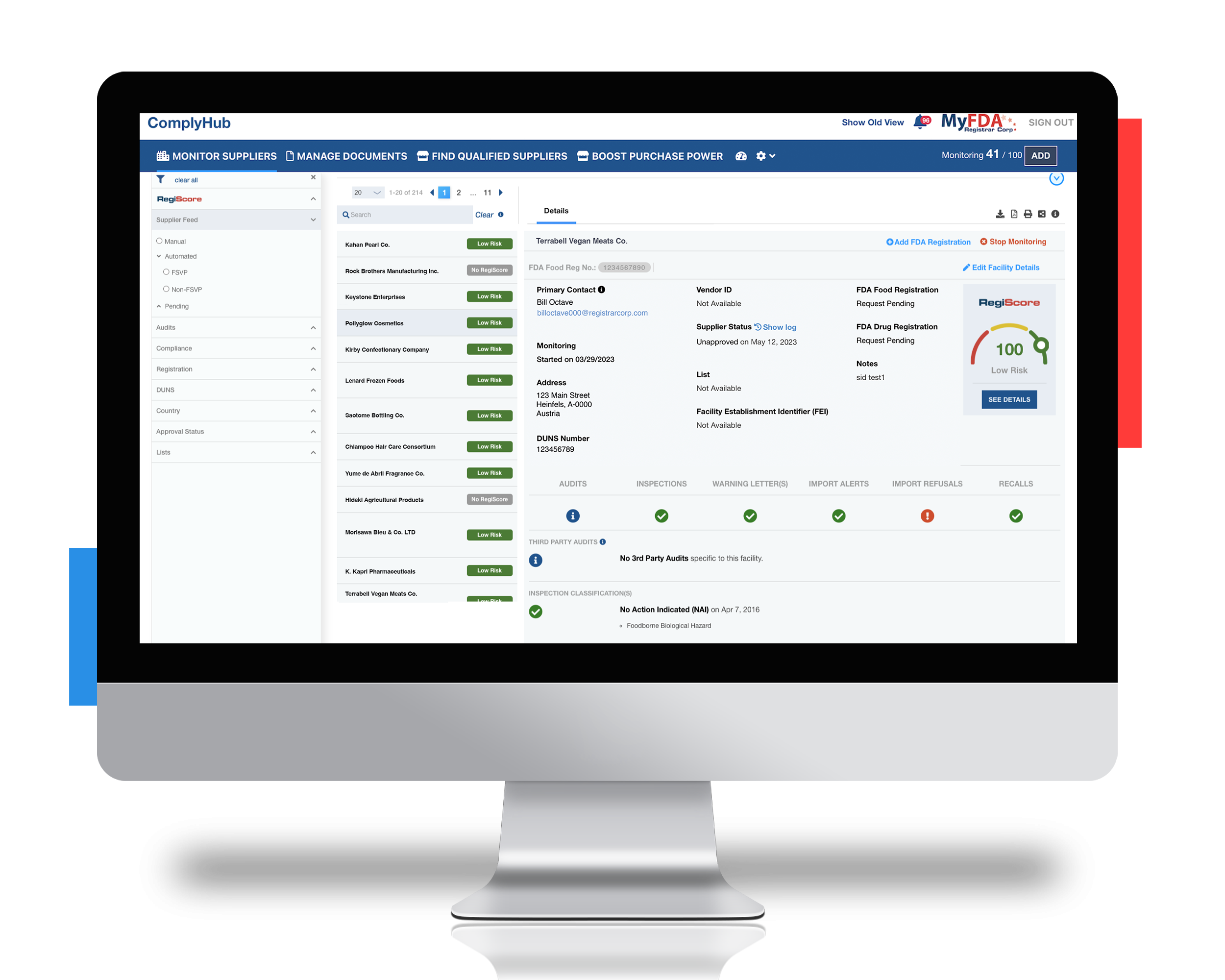
As part of an FSVP, importers must monitor all their suppliers and their facilities to ensure compliance with FDA. Without proper monitoring, this U.S. importer was at risk of paying detention charges averaging $3,000 higher for shipments coming through U.S. ports than the rest of the world. They were also unknowingly making these mistakes:
- Monitoring 2 supplier offices instead of production facilities
- Missing 12 suppliers from their FSVP
- Exposing themselves to risk including failed inspections, warning letters, and costly detentions


SOLUTION
Employing Registrar Corp’s technology and regulatory specialist team
By utilizing Registrar Corp’s tech-enabled services and ComplyHub solution, this importer was able to save time and money while avoiding costly penalties for non-compliance. Specifically, Registrar Corp helped:
- Save significant business hours by automating supplier data from the past 24 months
- Introduce client to our records management features —giving them access to our AI-powered document templates, automated deadline reminders, and more
- Avoid detentions by partnering with Registrar Corp’s regulatory specialists with 20+ years of experience
IMPACT
COSTS SAVED
$57,000
Saved yearly in labor to automate and maintain compliance
SUPPLIERS FOUND
12
Allowing for full FSVP compliance
FEES AVOIDED
$7,500
In detention fines saved by complying with FSVP
Stay FDA compliant 24/7