Read the updated information regarding the Food Traceability Rule in our new article.
The U.S. Food and Drug Administration (FDA) recently finalized an eighth rule under the Food Safety Modernization Act (FSMA) known as Requirements for Additional Traceability Records for Certain Foods (Food Traceability Final Rule). The Rule is intended to fulfill recordkeeping requirements under FSMA that help FDA quickly identify recipients of adulterated food.
Who Does This Affect?
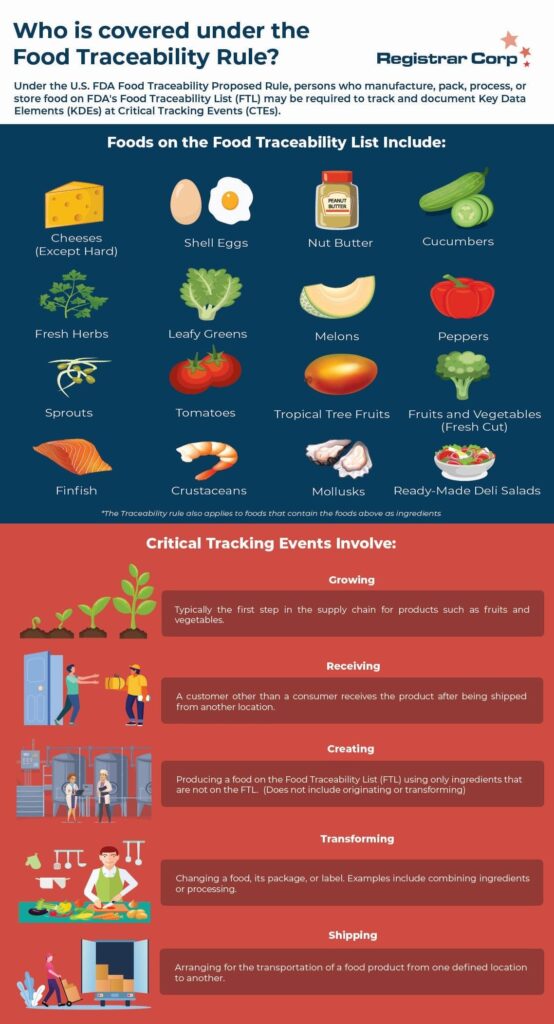
This rule applies to persons who manufacture, process, pack, or hold human food on FDA’s Food Traceability List (FTL), including retailers and distributors. Unlike many of the other FSMA rules, the Food Traceability Proposed Rule is not limited to facilities registered with FDA.
The FTL defines products that FDA has determined are at higher risk of carrying foodborne illnesses and thus will be subject to these additional tracking requirements. The Agency makes the important distinction that the rule applies “not only to the foods specifically listed, but also to any foods that contain listed foods as ingredients.”
FDA may add products to the FTL if they deem other foods to be high risk and require additional tracking. FDA only requires traceability records for products on the list, but they encourage companies to apply the requirements of the rule to other products they handle.
FDA has established exemptions for small farms and other small originators; farms that sell directly to consumers; handlers of produce products that are processed commercially in a way that adequately reduces the number of harmful microorganisms; and handlers of certain foods that are rarely consumed raw.
What Does This Rule Require?
The Food Traceability Proposed Rule requires covered persons to keep records of Key Data Elements (KDEs) for certain Critical Tracking Events (CTEs). FDA considers growing, receiving, creating, transforming, and shipping to be Critical Tracking Events.
Each Critical Tracking Event has different applicable Key Data Elements. For example, growers are required to record the coordinates of their growing area as a Key Data Element. The receivers of the product must establish records of quantity and measurements of the products, location identifiers established by the originator of the food, and more.
The first person to receive the product must establish a traceability lot code if they receive the food from an originator who has not already established one. Each subsequent person must maintain similar records of the product’s quantity and its identifier code.
Each covered person in the supply chain is required to maintain records of the Key Data Elements up until the point of a “kill step.” A kill step is defined by the FDA as a process that significantly minimizes pathogens found in the food. This could include cooking, high pressure processing, pasteurizing, and other processing activities. The person who implements the kill step must keep record of the step, as well as the Key Data Elements of previous Critical Tracking Events they conduct, but subsequent owners do not need to continue to track Key Data Elements. The kill step marks the end of the Key Data Element recordkeeping requirements of the rule.
In addition to requiring records of Key Data Elements, the proposed rule requires covered persons to establish and maintain traceability program records. These records are intended to help the FDA quickly and easily understand an entity’s traceability program. The traceability program records are meant to contain a “description of the reference records on which the firm maintains the required Key Data Elements.” These records also contain a list of which foods on the FTL are shipped by the entity, how their traceability lot codes are assigned, and other information on how the records can be better understood by FDA regulators.
Where Can I Keep the Records?
The proposed rule allows persons to keep the records in either electronic or original form, so long as they are kept in a manner that will prevent deterioration and loss. Persons must also maintain an electronic sortable spreadsheet containing the traceability information. Records need to be provided to FDA within 24 hours of the Agency requesting them. FDA requires covered persons to keep most records for two years from the date they create them.
How Do I Comply?
FDA allows persons subject to the to designate a third-party to establish and maintain the required records on their behalf. Registrar Corp can establish and maintain your Key Data Element records. Simply contact us for assistance.
Alternatively, Registrar Corp’s Document Management System (DMS) (available as an add-on for the ComplyHub) can simplify the process of developing and documenting your Key Data Elements. Persons covered under the Food Traceability Proposed Rule will find Registrar Corp’s DMS to be the perfect solution for requesting and storing required documentation for each of the products they handle.
Talk to our Regulatory Specialists today to learn more about how Registrar Corp can help by calling +1-757-224-0177 or emailing us at info@registrarcorp.com. You may also chat with a Regulatory Advisor 24-hours a day at www.registrarcorp.com/livechat








