Collect, Track, Report, and Download FDA-Compliant Adverse Event Submissions from One Secure, Convenient Platform
No more manual excel sheets, outdated processes, or worrying about the safety of your company’s trade secrets and private information. Adverse Event Management takes the guesswork out of managing every aspect of FDA’s adverse events requirements.
Collect customer adverse event submissions using branded URLs and QR codes.
Receive a branded URL and unique QR code to give your customers an easy, branded experience to submit any adverse events.

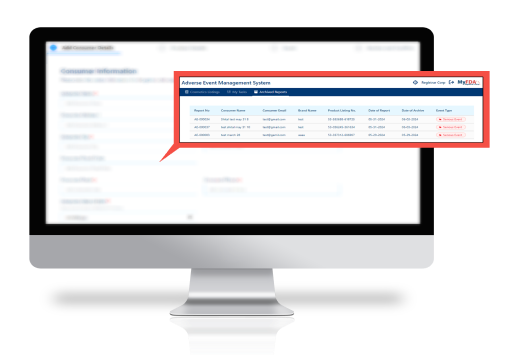
Track adverse and serious adverse events in real time
Monitor adverse event and serious adverse submissions in real time to improve your response time.
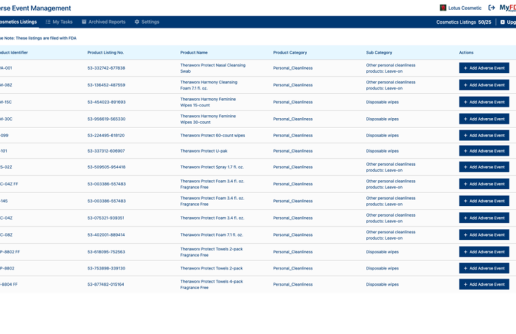
Connect adverse event submissions directly to your product listings.
Correlate adverse event submissions directly to your product listings to easily pull reports.

Download detailed,
FDA-compliant reports
for FDA submission.
Report adverse and serious adverse events to FDA with downloadable, FDA-compliant reports directly from Adverse Event Management.
Assure Good Standing with U.S. Retailers and FDA
Remain in good standing with U.S. FDA and your U.S. retailers with innovative software that manages and organizes your submissions according to FDA requirements and timelines.

All You Need to Know About Adverse Events
Learn from our cosmetic experts on how to navigate MoCRA adverse events reporting compliance.
Ready to take the next step?
To get started, submit this form to request demo, or buy now and start managing adverse events reporting immediately..



