CHALLENGE
24-hr timeline for FSVP compliance opened wholesale importer up to host of damaging consequences
When FDA conducts FSVP inspections, they mandate that documents must be “retrieved within 24 hours of request.” Without an FSVP or proper documentation, this tight timeline left a wholesale U.S. importer scrambling to comply and facing a multitude of issues, pending failure:
- Public Warning Letter issued by FDA causing significant reputation damage
- Detained shipments and penalties costing valuable time and resources
- Halted business operations due to compounding effect of noncompliance


SOLUTION
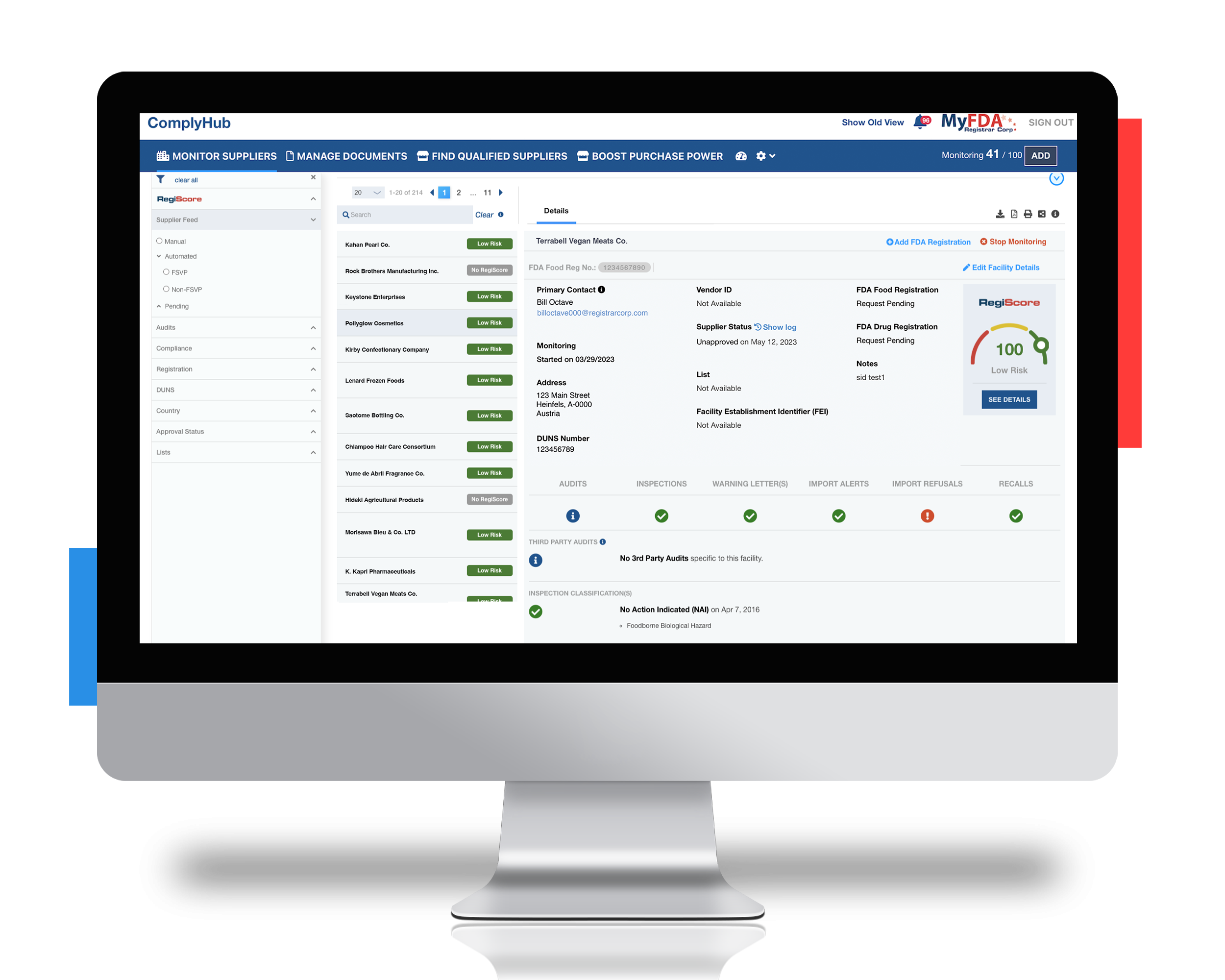
Leveraging Registrar Corp’s industry experts and automated FSVP compliance platform, ComplyHub
With guidance from our Food Safety experts and utilizing our ComplyHub solution, the importer avoided a failed inspection. Specifically, Registrar Corp helped to:
- Explain FSVP requirements and how to become compliant
- Consult on inspection specifics and how to properly respond to FDA requests
- Introduce ComplyHub’s supplier and document management features as a means of lowering long-term risk and satisfying FSVP requirements going forward
IMPACT
COSTS SAVED
$56,000
Saved per year in labor costs required to maintain FDA compliance
DETENTIONS AVOIDED
40+
Product categories free from potential detentions via ComplyHub
SUPPLIERS MONITORED
3
Import countries with all suppliers successfully monitored simultaneously
Stay FDA compliant 24/7