CHALLENGE
Warning Letter issued after FDA determines clients is missing 90+ suppliers in FSVP plan
After being issued a Warning Letter, a U.S. food importer was concerned about significant business repercussions and needed support. They had unknowingly misidentified their suppliers. As a result, their FSVP plan was noncompliant. With only 15 days to respond to FDA (or risk their shipments being detained), they needed to:
- Uncover why they were responsible for a supplier they didn’t know they had
- Properly identify any suppliers and update their FSVP plan accordingly
- Respond properly to the warning letter to avoid fines and detention at port
- Introduce proactive measures to prevent this from happening in the future


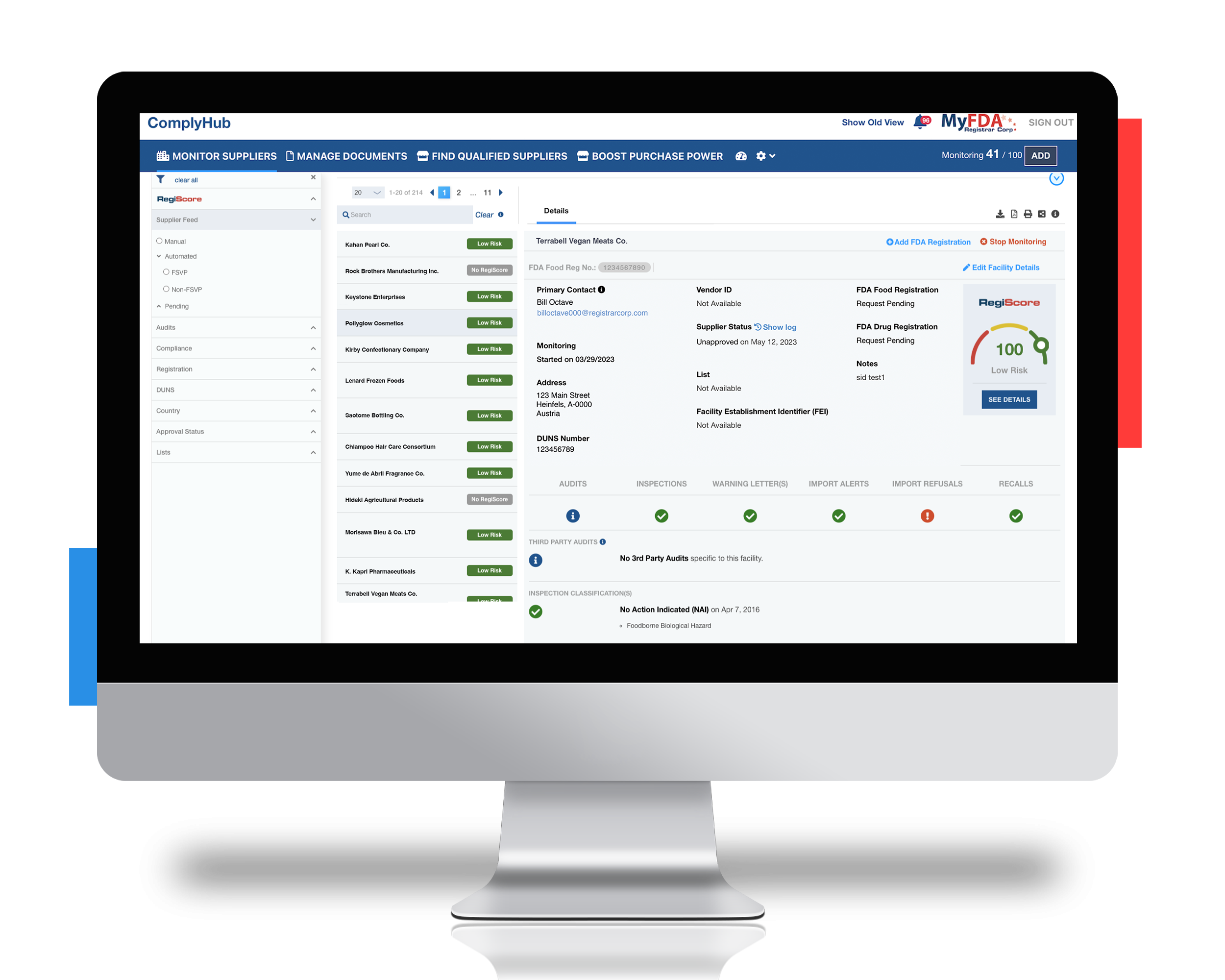
SOLUTION
Leveraging Registrar Corp’s industry experts and automated FSVP compliance software, ComplyHub
Through a combination of expert education and automated technology, Registrar Corp not only solved this importer’s immediate issues, but set them up for future success—avoiding costly fines and keeping their supply chain healthy. Specifically, Registrar Corp helped to:
- Explain the consequences of FSVP non-compliance at the FDA Warning Letter stage
- Provide access to our ComplyHub platform and its supplier tracking features, uncovering data they were obligated to track under FSMA
- Identify that they had been working with 90+ additional suppliers under an international distributor
- Address the Warning Letter and avoid significant business delays and losses
IMPACT
TIME SAVED
1,000s
Of hours gained through new efficiencies and streamlined compliance for 100+ suppliers
COSTS AVOIDED
$145,000+
Avoided in labor cost increases by training existing staff to use ComplyHub
FEES PREVENTED
$1,000+
Saved in potential fees from FDA by identifying all suppliers and updating FSVP plans
Stay FDA compliant 24/7