With an increasing reliance on imported foods, ensuring the safety of these products is crucial for protecting US consumers. The Foreign Supplier Verification Program (FSVP), established under the FDA’s Food Safety Modernization Act (FSMA), plays a key role in safeguarding the food supply chain by setting rigorous standards for importers. FSVP compliance requires US importers to verify that their foreign suppliers meet the same stringent food safety standards as domestic producers.
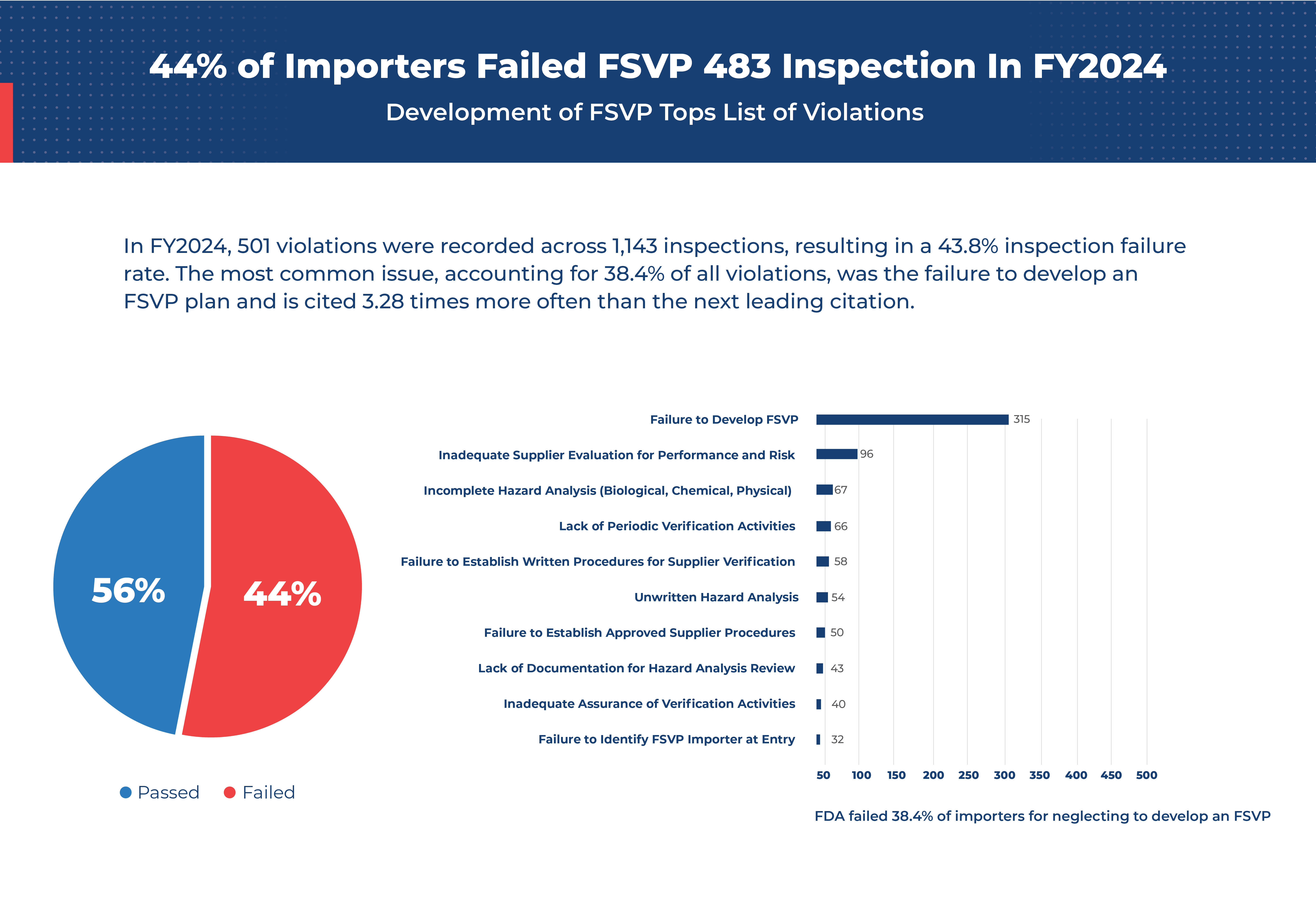
In Fiscal Year 2024, FSVP violations impacted 43.8% of inspections, with the most common issues stemming from a failure to establish a basic FSVP.
This article will explore what FSVP violations are, how they happen, their potential impact on importers, and the steps importers can take to avoid or quickly resolve them. We’ll also highlight the top FSVP violations reported in FY2024, offering insights into the areas where importers should focus their compliance efforts.
Jump to your preferred section:
- What Are FSVP Violations?
- How Do They Happen?
- Top Violations of FY2024
- FSVP Violation Impact
- How to Prevent Violations
- How to Quickly Recover
- Ensuring FSVP Compliance
What Are FSVP Violations? Understanding Their Implications
FSVP violations refer to any instance where a US importer fails to meet the requirements outlined in the FSVP rule. The purpose of these requirements is to ensure that food products entering the US are safe, properly labeled, and produced in compliance with FDA regulations.
When importers neglect to establish or follow the necessary verification activities, they become susceptible to FSVP violations.
FSVP violations typically fall into specific categories, such as failing to conduct a hazard analysis, omitting supplier evaluations, or inadequately documenting verification activities. Understanding these violations and their implications is essential for importers looking to maintain compliance and avoid costly penalties.
In FY2024, 501 violations were recorded across 1,143 inspections, resulting in a 43.8% inspection failure rate. The most common issue, accounting for 38.4% of all violations, was the failure to develop an FSVP plan and is cited 3.28 times more often than the next leading citation.
“The most common FSVP violation in FY2024—accounting for 38.4% of cases—was a complete failure to develop an FSVP plan.”
How Do FSVP Violations Happen? Common Causes
FSVP violations typically occur when importers fail to meet one or more requirements set by the FDA. These requirements, while straightforward in theory, can be challenging to implement consistently across diverse supply chains. Understanding how violations happen can help importers recognize potential weak points in their own compliance practices and take corrective actions before issues arise. Here are some common reasons why FSVP violations occur:
Lack of Awareness of FSVP Requirements
Many violations stem from a lack of understanding or knowledge about FSVP regulations. New importers or businesses unfamiliar with FDA requirements may not realize the extent of the documentation, supplier verification, and monitoring needed to achieve compliance. Without proper training or guidance, these importers are at a higher risk of inadvertently neglecting essential compliance steps.
Insufficient Resources & Staff Training
Compliance with FSVP requires dedicated resources, including trained personnel and adequate funding. Smaller businesses or those with limited resources may struggle to allocate staff or budget toward developing and maintaining an FSVP. Insufficient training for employees handling FSVP responsibilities can lead to incomplete documentation, improper evaluations, and missed verification activities.
Complex Supply Chains & Supplier Relationships
Food importers often work with a variety of foreign suppliers, each with its own production processes and safety practices. Managing complex supply chains makes it harder to conduct consistent, thorough evaluations and ensure each supplier is compliant. Miscommunications, language barriers, and differing standards across countries further complicate the verification process, increasing the risk of non-compliance.
Neglecting Documentation & Record-Keeping
Documentation is a cornerstone of FSVP compliance, as the FDA requires detailed records of supplier evaluations, hazard analyses, and verification activities. Some importers may focus on meeting safety standards but overlook the importance of thorough documentation. In the FDA’s view, if it’s not documented, it didn’t happen. Missing records or incomplete documentation are among the most frequent causes of violations, particularly when it comes to hazard analyses and verification procedures.
“In the FDA’s view, if it’s not documented, it didn’t happen.”
Failure to Adapt to Changes in Suppliers or Regulations
FSVP compliance is an ongoing process. Importers need to re-evaluate suppliers periodically and update their FSVP when new information, hazards, or regulatory changes arise. Some importers fail to monitor these changes actively, which can result in outdated FSVPs that no longer meet FDA standards. Supplier changes, such as new food products or altered production processes, also necessitate updates to the FSVP.
Over-Reliance on Suppliers for Compliance
While foreign suppliers are responsible for adhering to certain standards, importers ultimately bear the burden of proving compliance to the FDA. Some importers may mistakenly assume their suppliers’ safety practices are adequate without conducting independent verifications. Over-relying on suppliers for compliance documentation, without verifying accuracy or consistency, often leads to citations.
By understanding the factors that contribute to FSVP violations, importers can take proactive measures to strengthen their compliance efforts. In the following section, we’ll discuss the impact of these violations and why adhering to FSVP requirements is crucial for food importers.
Top FSVP Violations in FY2024: Insights & Lessons
In FY2024, the FDA’s data revealed the most frequent FSVP violations, indicating areas where importers struggled to meet regulatory standards. Here’s a breakdown of the top violations and what each entail:
1. Failure to Develop an FSVP (38.4%)
This violation occurs when importers do not create an FSVP plan at all. The FSVP plan outlines how an importer will verify that the food they import is safe and meets US food safety standards. Without this foundational document, importers are immediately at risk of non-compliance.
2. Inadequate Supplier Evaluation for Performance and Risk (11.7%)
Importers must assess the performance and risk level of each supplier, reviewing factors such as the supplier’s compliance history, food safety practices, and any past violations. Skipping this evaluation puts consumers at risk and leaves the importer non-compliant.
3. Incomplete Hazard Analysis (Biological, Chemical, Physical) (8.2%)
A thorough hazard analysis is essential to identify potential risks in the food product, such as contamination by pathogens, chemicals, or physical hazards. Failure to conduct a complete hazard analysis compromises safety and leads to non-compliance.
Block Quote: “Inadequate supplier evaluation and incomplete hazard analysis are common gaps leading to FSVP violations.”
4. Lack of Periodic Verification Activities (8%)
Importers are required to perform regular verification activities, like audits,record reviews and sampling and testing, to ensure ongoing compliance of their foreign suppliers. Without periodic checks, importers cannot confirm that suppliers continue to meet safety standards.
5. Failure to Establish Written Procedures for Supplier Verification (7.1%)
Written verification procedures help importers consistently evaluate their suppliers. Without written protocols, there’s a lack of accountability and a higher likelihood of safety issues going unnoticed.
6. Unwritten Hazard Analysis (6.6%)
A hazard analysis must be written to provide a clear record of identified risks and the controls in place to mitigate them. Failure to write out this analysis can lead to oversight of critical safety measures and gaps in the identification of necessary verification activities.
7. Failure to Establish Approved Supplier Procedures (6.1%)
Importers must have written procedures for selecting and approving suppliers to ensure they only work with those who meet FDA standards. Without these procedures, it’s challenging to verify a supplier’s compliance.
8. Lack of Documentation for Hazard Analysis Review (5.2%)
Importers are required to review and document their suppliers’ hazard analyses to demonstrate thorough risk management and make a determination of compliance. When importers don’t keep these records, they fall short of compliance standards.
9. Inadequate Assurance of Verification Activities (4.9%)
Importers must provide evidence that verification activities (such as testing or auditing) are effective. Failure to document and prove these activities leaves them at risk of citation.
10. Failure to Identify FSVP Importer at Entry (3.8%)
The FSVP importer must be clearly identified when food products enter the US to ensure accountability. If inaccurate information is provided at entry, it raises serious concerns over traceability and safety.
The Impact of FSVP Violations: Consequences for Your Business
FSVP violations carry serious consequences, impacting not only the importers but also the wider supply chain and consumer trust. When an importer fails to meet FSVP requirements, it creates potential safety risks for US consumers, as imported food may not meet the necessary standards for quality and safety. Violations can result in significant financial and operational repercussions for businesses, along with potential legal consequences.
Regulatory Consequences & Financial Penalties
The FDA takes FSVP violations seriously. Importers who fail to comply with FSVP standards may face regulatory actions, including Warning Letters, import alerts, and even product seizures. In extreme cases, the FDA may also suspend the facility’s registration, preventing further imports until compliance is restored. These actions not only disrupt business operations but can also lead to substantial financial penalties.
Damage to Business Reputation
Non-compliance with FSVP regulations can severely harm a company’s reputation, as consumers, suppliers, and partners may lose confidence in the importer’s ability to maintain safety standards. In a competitive market, where consumer trust is paramount, even a single FSVP violation can lead to lasting damage to brand integrity and consumer loyalty.
“Non-compliance with FSVP regulations can severely harm a company’s reputation, damaging brand integrity and consumer trust.”
Increased Scrutiny & Compliance Costs
Importers who have been cited for FSVP violations often face increased scrutiny in subsequent inspections. The FDA may inspect these businesses more frequently or impose additional compliance requirements, which can increase operational costs. Additionally, the process of rectifying violations and implementing corrective actions to meet FDA standards can be costly, both in terms of time and resources.
By understanding the potential impacts of FSVP violations, importers can better appreciate the importance of maintaining compliance. Consistent adherence to FSVP requirements helps to protect both consumers and the importer’s business interests.
How to Prevent FSVP Violations: Key Strategies
Preventing FSVP violations requires a proactive approach to ensure that every aspect of the Foreign Supplier Verification Program is in place and meticulously followed. The following strategies can help importers safeguard their compliance and reduce the risk of costly violations:
Develop a Comprehensive FSVP Plan
A well-documented and comprehensive FSVP plan is the foundation for compliance. This plan should outline every requirement under FSVP, detailing the procedures for verifying foreign suppliers, conducting risk assessments, performing hazard analyses, and setting verification activities. Regularly review and update the plan to ensure it aligns with FDA requirements and current practices.
“A comprehensive FSVP plan is the foundation for compliance—outline every requirement, from supplier verification to hazard analysis.”
Conduct Thorough Supplier Evaluations
A critical component of FSVP compliance is assessing each supplier’s ability to meet FDA food safety standards. Importers should examine a supplier’s compliance history, including previous FDA inspections, food safety practices, and any past violations. Establishing a process for supplier evaluation and ongoing monitoring ensures that suppliers continue to uphold the necessary standards.
Utilize Qualified Individuals for FSVP Activities
Designating a Qualified Individual (QI) who understands FSVP requirements and FDA regulations is instrumental in maintaining compliance. The QI should oversee FSVP activities, including supplier verification, hazard analysis, and risk assessment. Training and certifying individuals responsible for FSVP tasks can prevent oversight and ensure that compliance measures are accurately followed.
“Utilizing Qualified Individuals for FSVP activities helps ensure accuracy in verification and compliance practices.”
Perform Regular Hazard Analyses
Conducting hazard analyses considering the food, the supplier’s process and their history will identify potential biological, chemical, and physical hazards requiring verification. A thorough hazard analysis provides a solid basis for implementing appropriate verification activities to prevent safety issues in the food supply.
Document Verification and Monitoring Activities
Maintaining accurate and complete records is crucial to demonstrate FSVP compliance. Each supplier’s verification activities, corrective actions, and other related procedures must be documented and organized in an accessible manner. Regularly update records to reflect any changes in supplier processes or product formulations impacting the FSVP determinations and be prepared to present these records in the event of an FDA inspection.
Schedule Routine Audits (Internal or External)
Performing regular audits, whether internal or through third-party entities, helps verify that FSVP procedures are being followed correctly. Routine audits can identify areas of improvement, potential risks, and any lapses in compliance, enabling you to address issues before they lead to violations.
How to Quickly Recover from FSVP Violations
In the event of an FSVP violation, swift and strategic action is essential to regain compliance and minimize the impact on your business. Here’s a step-by-step guide on how to recover from FSVP violations effectively:
1. Address the Violation Immediately
Once a violation is identified, whether by an FDA inspection or internal audit, take immediate steps to address the issue. This may involve halting imports from the specific supplier until corrective actions are in place or modifying existing procedures to ensure compliance with FSVP requirements.
2. Conduct a Root Cause Analysis
Understanding the root cause of the violation is essential to prevent future occurrences. Perform a detailed analysis to identify what led to the violation—such as gaps in supplier evaluation, inadequate hazard analysis, or lapses in documentation. Corrective actions should be tailored to address these specific issues.
“Address FSVP violations immediately and conduct a root cause analysis to prevent future issues.”
3. Implement Corrective Actions
Based on the findings from the root cause analysis, implement corrective actions to address compliance gaps. This may include updating FSVP procedures, retraining staff, or enhancing verification activities. Ensure that corrective actions are thoroughly documented, as this will demonstrate to the FDA your commitment to compliance.
4. Strengthen Supplier Verification and Monitoring
FSVP violations often highlight weaknesses in supplier verification and monitoring. Strengthen these areas by conducting additional verification activities, such as more frequent on-site audits, product testing, or record reviews. Re-evaluate high-risk suppliers and establish stricter controls to ensure they meet FDA requirements.
5. Update and Maintain Records
Accurate record-keeping is crucial for demonstrating compliance during future inspections. Update all relevant records to reflect the corrective actions taken, the results of root cause analyses, and any updates to FSVP procedures. Maintaining organized and accessible records will facilitate smoother future audits and inspections.
6. Consider Third-Party Support for Compliance
If FSVP compliance is complex or resource-intensive, consider engaging third-party support to navigate regulatory requirements. Companies like Registrar Corp offer FSVP compliance services, providing expertise in FSVP plan development and implementation, supplier verification, and documentation management. Third-party support can streamline recovery efforts and help prevent future violations.
“By proactively developing a robust FSVP plan, importers can minimize the risk of violations and protect the food supply chain.”
Securing FSVP Compliance for a Safer Food Supply Chain
Maintaining compliance with the Foreign Supplier Verification Program is essential for protecting both consumers and your business from the risks associated with imported food products. By proactively developing a robust FSVP plan, conducting thorough supplier evaluations, and meticulously documenting verification activities, importers can minimize the risk of violations and contribute to a safer food supply chain in the United States.
However, navigating FSVP requirements can be challenging, especially given the intricacies of FDA regulations and the high standards expected. Identifying and managing corrective actions for supplier non-compliance is particularly arduous. Partnering with a trusted compliance expert like Registrar Corp can ease this burden.
With specialized knowledge in FDA regulations and a proven track record, Registrar Corp provides comprehensive support, including FSVP plan development, supplier verification, and ongoing compliance management.
By working with Registrar Corp, you can rest assured that your FSVP procedures are up to date, compliant, and ready to withstand FDA scrutiny, allowing you to focus on growing your business with confidence in your compliance efforts.
Taking proactive steps now can prevent costly violations in the future, protect public health, and strengthen your business’s reputation in the global marketplace.
Embrace the tools, expertise, and support available to build a resilient FSVP process—securing your place in a compliant, safer food supply chain.